CITI Human Subjects Training
***ATTENTION INVESTIGATORS*** Current MU CITI Training is required for all new submissions. Lack of appropriate training will delay the review/approval of any new submissions.
Certification of human subjects research training is required for all principal investigators (PI), student principal investigators, and faculty advisers. Additionally, anyone serving as a member of the research team engaged in any of the following activities should complete training as well:
- obtaining information about living individuals by intervening or interacting with them for research purposes;
- obtaining identifiable private information about living individuals for research purposes;
- obtaining the voluntary informed consent of individuals to be subjects in research; and
- studying, interpreting or analyzing identifiable private information or data for research purposes.
Marquette offers human subjects research training through the Collaborative Institutional Training Initiative (CITI). CITI provides web-based training on a variety of research topics whose user base include thousands of institutions and users.
Each learner group contains 10-12 required modules and requires the completion of a brief 3-4 question quiz. Each module may take 10-20 minutes to complete (typically 1.5-2 hours total), and most modules contain a quiz. The modules do not have to be completed all in one session. A minimum cumulative score of 80% is required to pass the training.
1. The CITI is found at: https://www.citiprogram.org/
2. Register for an account if you do not already have one. If you already have an account (or newly created one), log in.
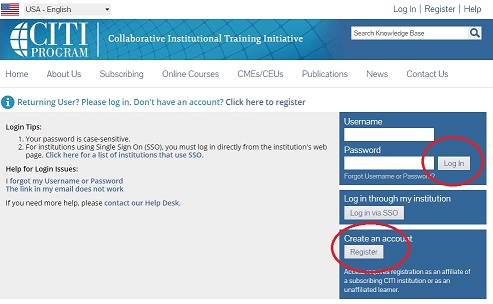
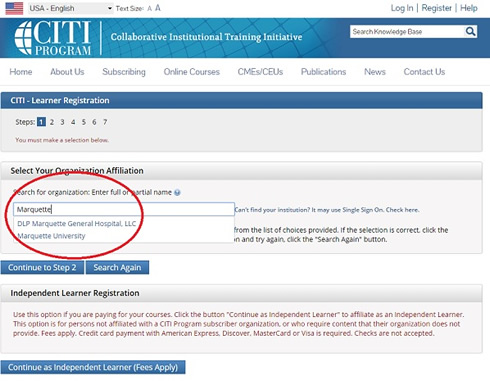
3. Under “Organization Affiliation” search for Marquette University and complete the registration process.
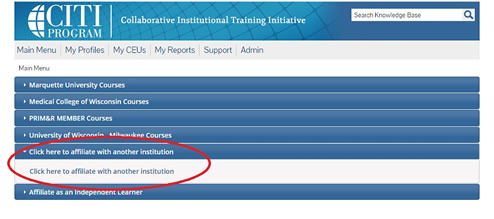
4. If you are currently registered with CITI, after logging in, from the main menu, affiliate your account with Marquette University.
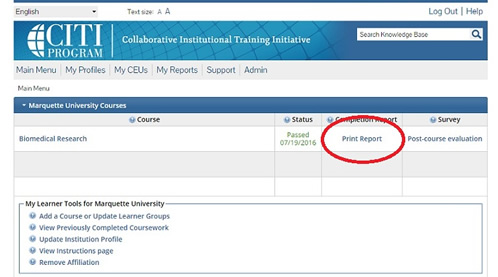
Marquette IRB automatically receives an email notification for each human subjects completion. However, a completion report is accessible by the user by clicking "Print Report" and saving as a pdf from the main menu.
If you would like to know if an individual has completed CITI training, please email the Office of Research Compliance at orc@marquette.edu.
D1. "Protecting Human Research Participants," sponsored by the NIH Office of Extramural Research. This training tutorial takes approximately 2 hours to complete.
The Office of Human Research Protections (OHRP) offers the pamphlet "Becoming a Volunteer: It's Your Decision." These pamphlets are available in English and Spanish. Contact Marquette's Office of Research Compliance if you would like a hard copy of this pamphlet. If you would like an electronic copy, follow the links below.
Becoming a Research Volunteer: It's Your Decision (PDF)- Follow this link to access the document.
Becoming a Research Volunteer: It’s Your Decision (in Spanish) (PDF)- Follow this link to access the document.




