Dr. Marieke Gilmartin's Research
Visit the Gilmartin Lab Website for the latest updates about our research and to meet our team.
Interested in joining our research team? Email Dr. Gilmartin to see if positions are available.
One of the most intriguing questions in neuroscience is how individual sensory, temporal, and emotional components of an experience are encoded into rich, persistent memories. Not only do these memories make up our personal narrative, but they are central to adaptive behavior. Healthy emotional learning allows us to predict and avoid danger and approach reward and safety. A distributed network of cortical and subcortical brain areas participate in successful memory formation, and dysfunction in these circuits leads to maladaptive behavior observed in a number of mental health disorders, including post-traumatic stress disorder (PTSD) and other anxiety disorders, schizophrenia, and addiction. Our work seeks to determine how prefrontal cortex, hippocampus, and amygdala functionally interact to form, store, and retrieve emotional memory.
Current research questions in our lab include:
- How do prefrontal cortical regions participate in memory formation and retrieval?
- How does prefrontal cortex interact with other learning and emotional brain systems to regulate memory and behavior?
- How do females and males differentially engage these networks to form and express fear memories?
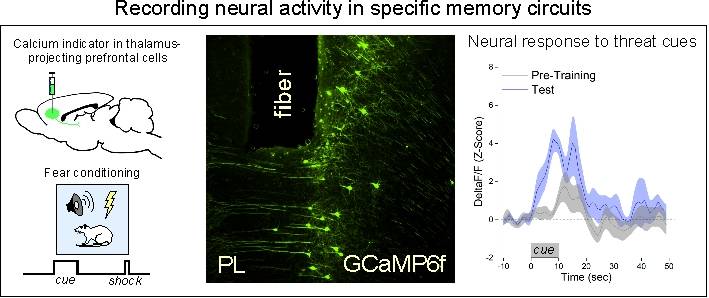
To address these questions, we use a combination of behavioral, systems, and molecular approaches to examine the neurobiology of learning and memory. Immunohistochemistry and targeted injections of pharmacological agents into individual brain regions allow us to examine the intracellular signaling pathways activated by learning or memory retrieval. Multi-channel single-unit electrophysiology, in-vivo calcium imaging, and optogenetic tools allow us to record and directly manipulate unit firing with temporal and circuit specificity.
Selected Publications
- Herbst, M.R., Twining, R.C., Gilmartin, M.R. (2022) Ventral hippocampal shock encoding modulates the expression of trace cued fear. Special Issue: Tribute to David Bucci in Neurobiology of Learning and Memory.
- Gilmartin, M.R. and Ferrara, N.C. (2021) Pituitary adenylate cyclase-activating polypeptide (PACAP) in learning and memory. Frontiers in Cellular Neuroscience. 15. https://doi.org/10.3389/fncel.2021.663418
- Kirry, A.J., Twining, R.C., Gilmartin, M.R. (2020) Prelimbic input to basolateral amygdala facilitates the acquisition of trace cued fear memory under weak training conditions. Neurobiology of Learning and Memory. 172. https://doi.org/10.1016/j.nlm.2020.107249
- Twining, R.C., Lepak, K., Kirry, A.J., Gilmartin, M.R. (2020) Hippocampal input to the prefrontal cortex mediates contextual, but not cued, fear memory in trace conditioning. Journal of Neuroscience. 40(16):3217-3230. https://doi.org/10.1523/JNEUROSCI.1453-19.2020
- Kirry, A.J., Herbst, M.R., Poirier, S.E., Maskeri, M.M., Rothwell, A.C., Twining, R.C., & Gilmartin, M.R. (2018) Pituitary adenylate-cyclase activating-polypeptide (PACAP) signaling in the prefrontal cortex modulates cued fear learning, but not spatial working memory, in female rats. Neuropharmacology. 133:145-154. https://doi.org/10.1016/j.neuropharm.2018.01.010
- Gilmartin, M.R., Balderston, N.L., & Helmstetter, F.J. (2014) Prefrontal cortical regulation of fear learning. Trends in Neurosciences. 37(8):455-464. http://dx.doi.org/10.1016/j.tins.2014.05.004
- Gilmartin, M.R., Miyawaki, H., Helmstetter, F.J., & Diba, K. (2013) Prefrontal cortex links non-overlapping events in memory. Journal of Neuroscience. 33(26): 10910-10914.
- Gilmartin, M.R., Helmstetter, F.J. (2010) Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learning and Memory. 17:289-296.
- Gilmartin, M.R., McEchron, M.D. (2005) Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behavioral Neuroscience119(6):1496-1510.



